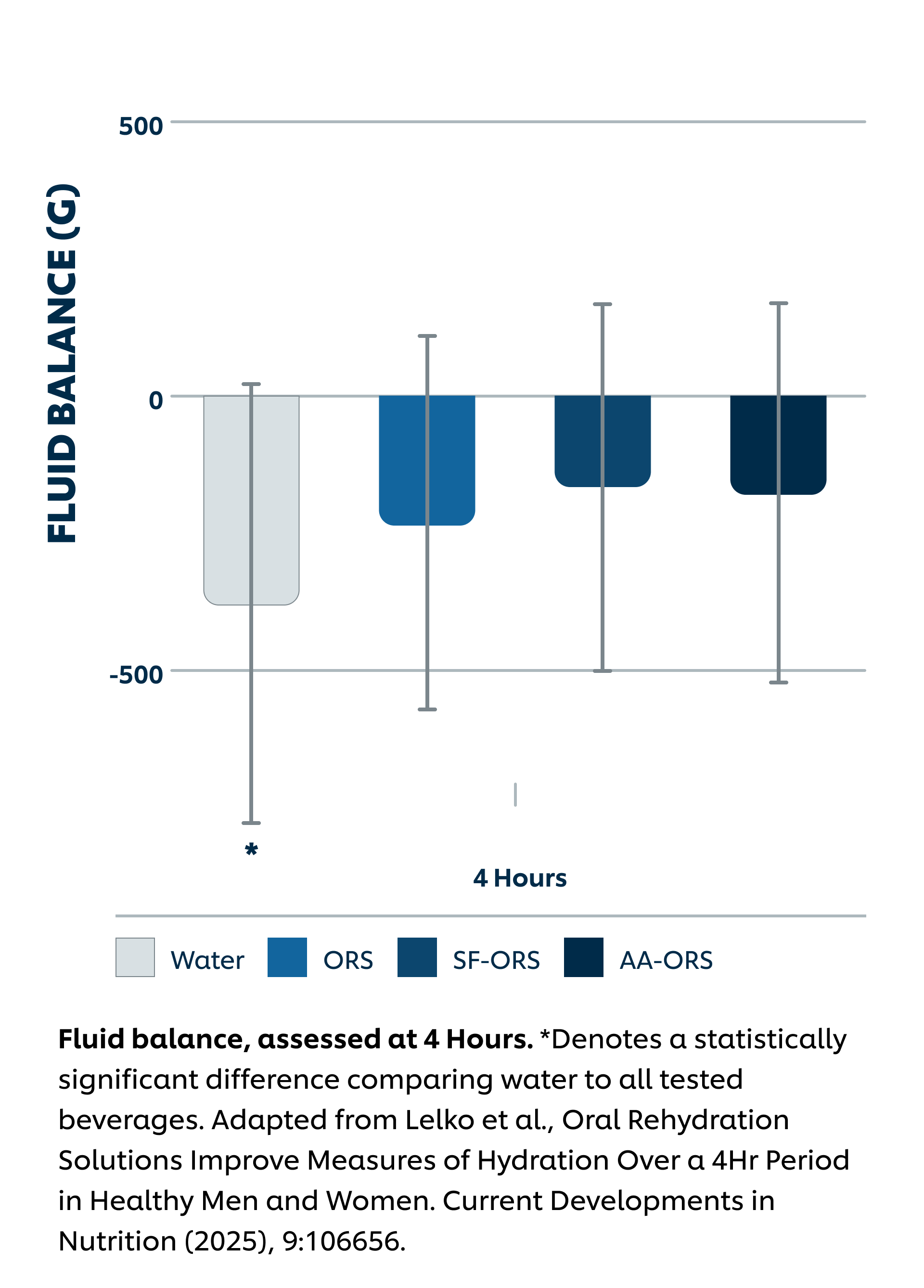
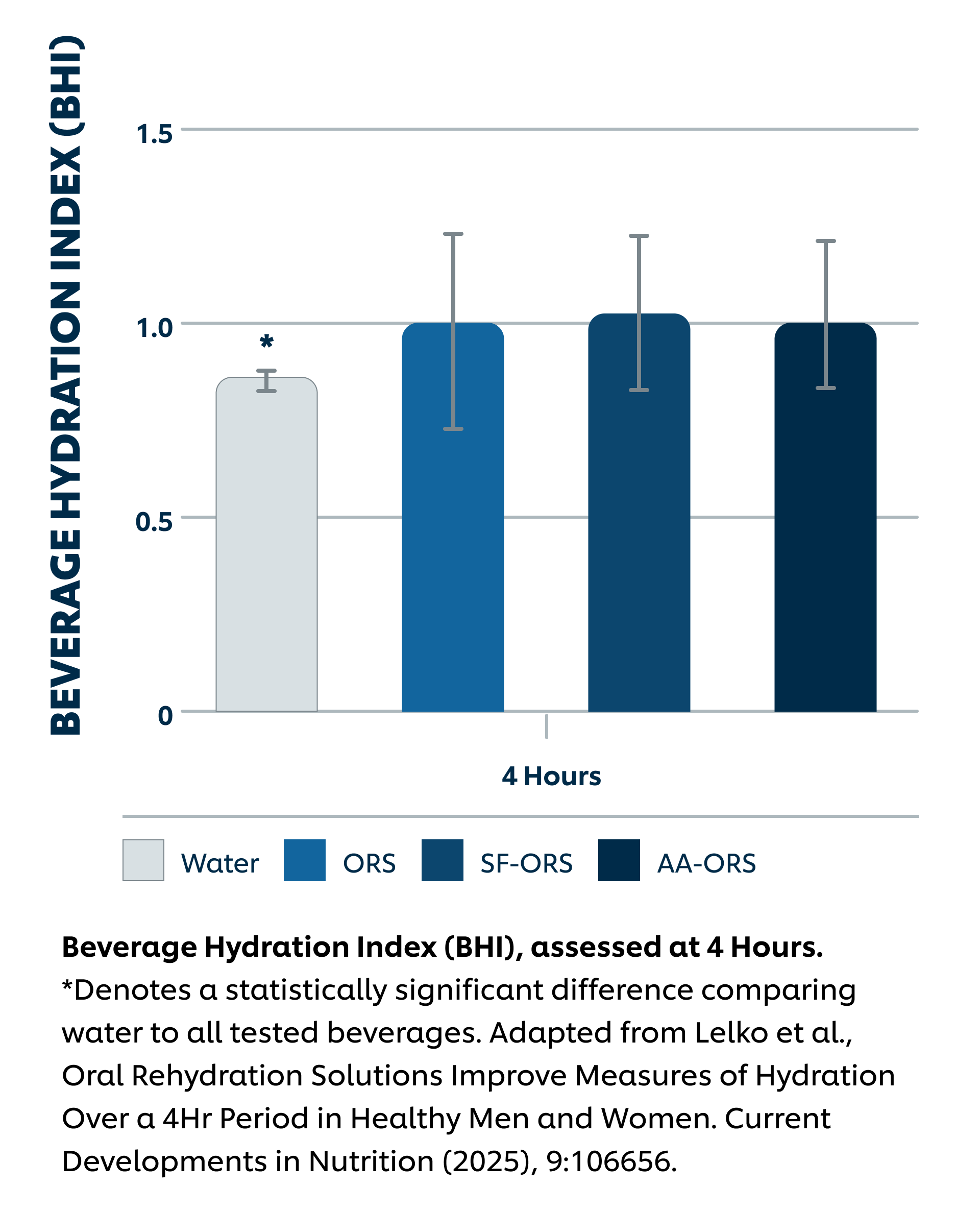
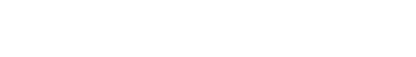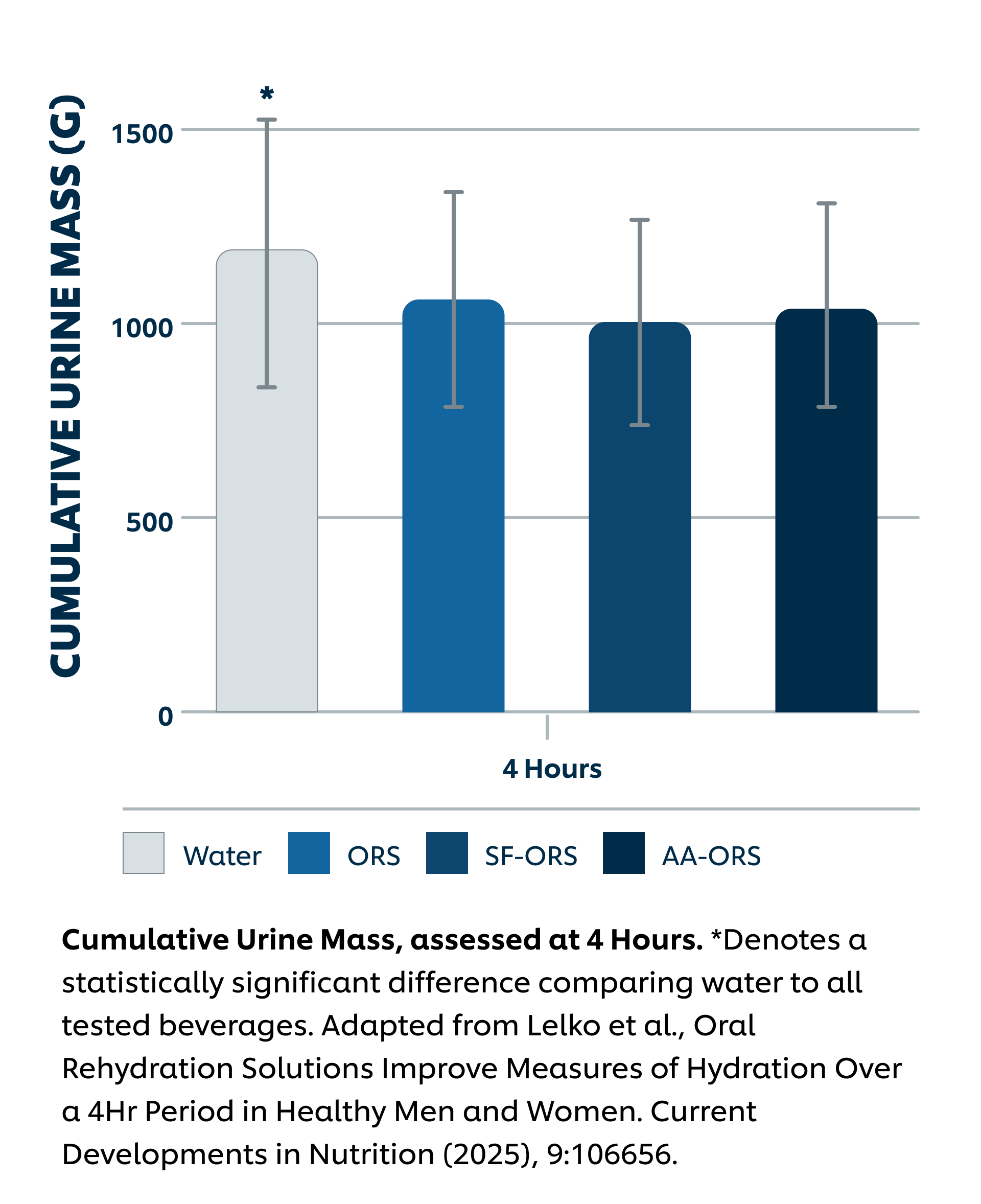Intervention: Sugar-Sweetened ORS (SS-ORS, Liquid I.V. Hydration Multiplier), Sugar-Free Allulose-Sweetened ORS (SF-ORS, Liquid I.V. Sugar-Free (with allulose)), Sugar-Free Allulose-Sweetened ORS (SF-ORS*, Liquid I.V. Sugar-Free (with amino acids)), or water. All participants (n = 28) completed the three active arms and the water arm of the protocol in a crossover study design. Each day, participants consumed 1L of the four beverages and urine was collected at the end of hours 1-4 post-intake.
Study Duration: 4 hours
Eligibility Criteria:
1. Healthy male and female participants, aged 18 - 45 years.
2. Consumes 2 L/day (female) or 2.5 L/day (male) water prior to study.
3. Able to fast for ~15 hours prior to each visit.
For more, plus the exclusion criteria, see
ClinicalTrials.gov